Usability Starts Here.®
We are Human Factors Experts.
We conduct quality research to guide the user-centered design of your healthcare products and improve usability, safety, and appeal.
ANSWER QUESTIONS YOU DIDN’T KNOW YOU HAD
Our research-driven approach provides the answers you need. From initial user needs through the evaluation process to regulatory submission, we're committed to helping you every step of the way.
200+
Validation studies completed
(in the last 5 years)
37,000+
Participants in
our database
WE ARE NOT TRADITIONAL CONSULTANTS
We are your human factors partners. Our team of human factors researchers collaborates with you, providing guidance through every project phase. Together, we uncover valuable insights that optimize the usability and safety of your products.
What We Do
USER NEEDS IDENTIFICATION
The most successful solutions are born from user needs. We observe, interview, and conduct workshops with actual users, so that you can gain a more accurate understanding of your users, their environments, and key tasks.
EXAMPLES
Field Research & Contextual Inquiry
Design Thinking Workshops
Acceptability Studies
REQUIREMENTS DEFINITION
Whether you are in the concept phase or looking to inform design specifications, focused research with our customized data capture tools can strengthen any project.
EXAMPLES
Eye Tracking
Human Capability Studies (e.g., Force Studies)
CONCEPT DEVELOPMENT
Both our in-house designers and engineers are human factors experts. We utilize their experience to consider all aspects of the user interface to bolster and transform our clients' ideas into realistic solutions.
EXAMPLES
Labeling Development (e.g., IFUs)
Packaging Development
GUI Development
ANALYTICAL METHODS
We use our vast experience of past healthcare products, along with market-available data, to analyze your product, no matter what phase it's in. We ensure you are in the best position possible to take the next steps.
EXAMPLES
Expert Reviews
Threshold Analysis
Competitive Benchmarking
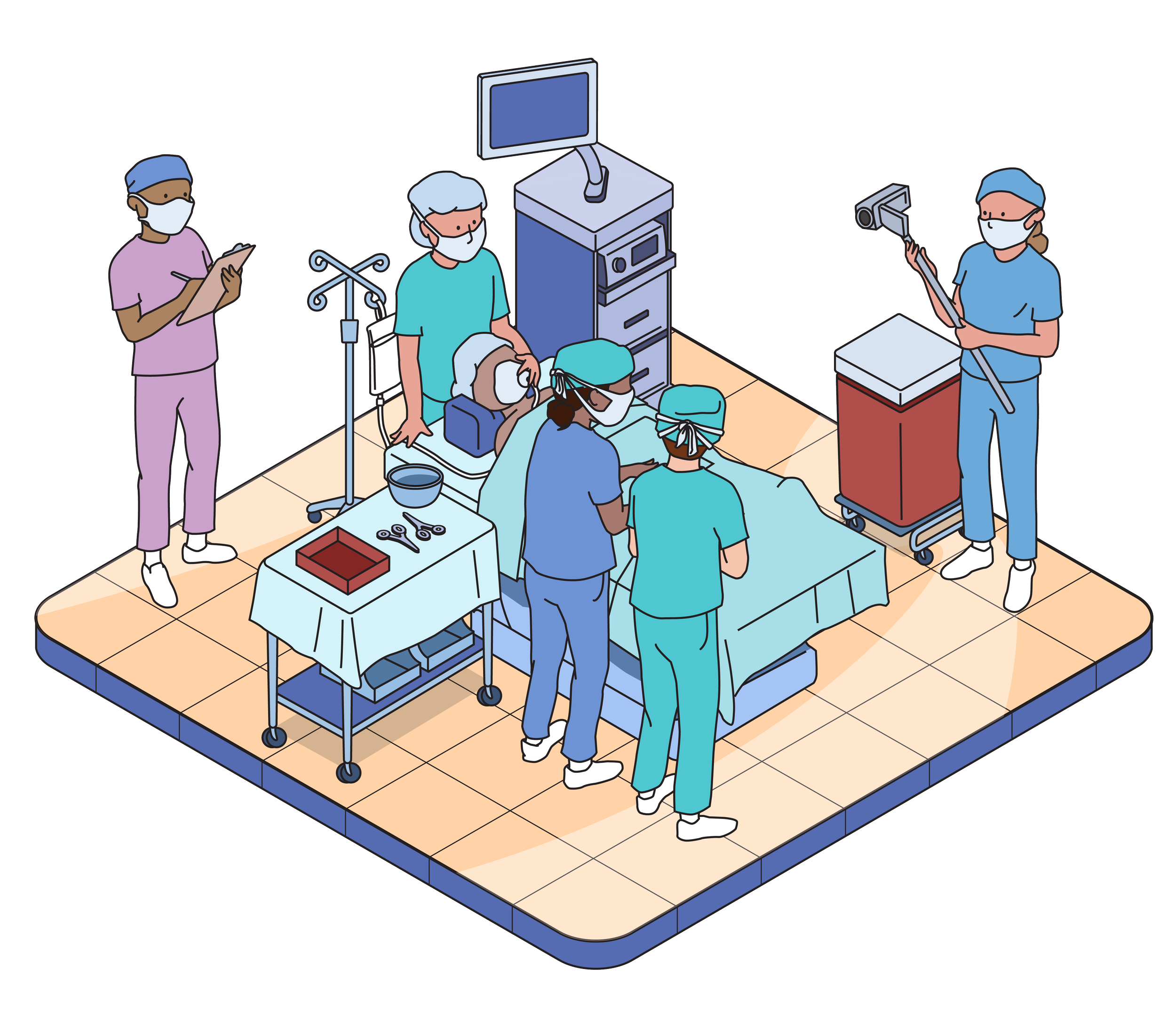
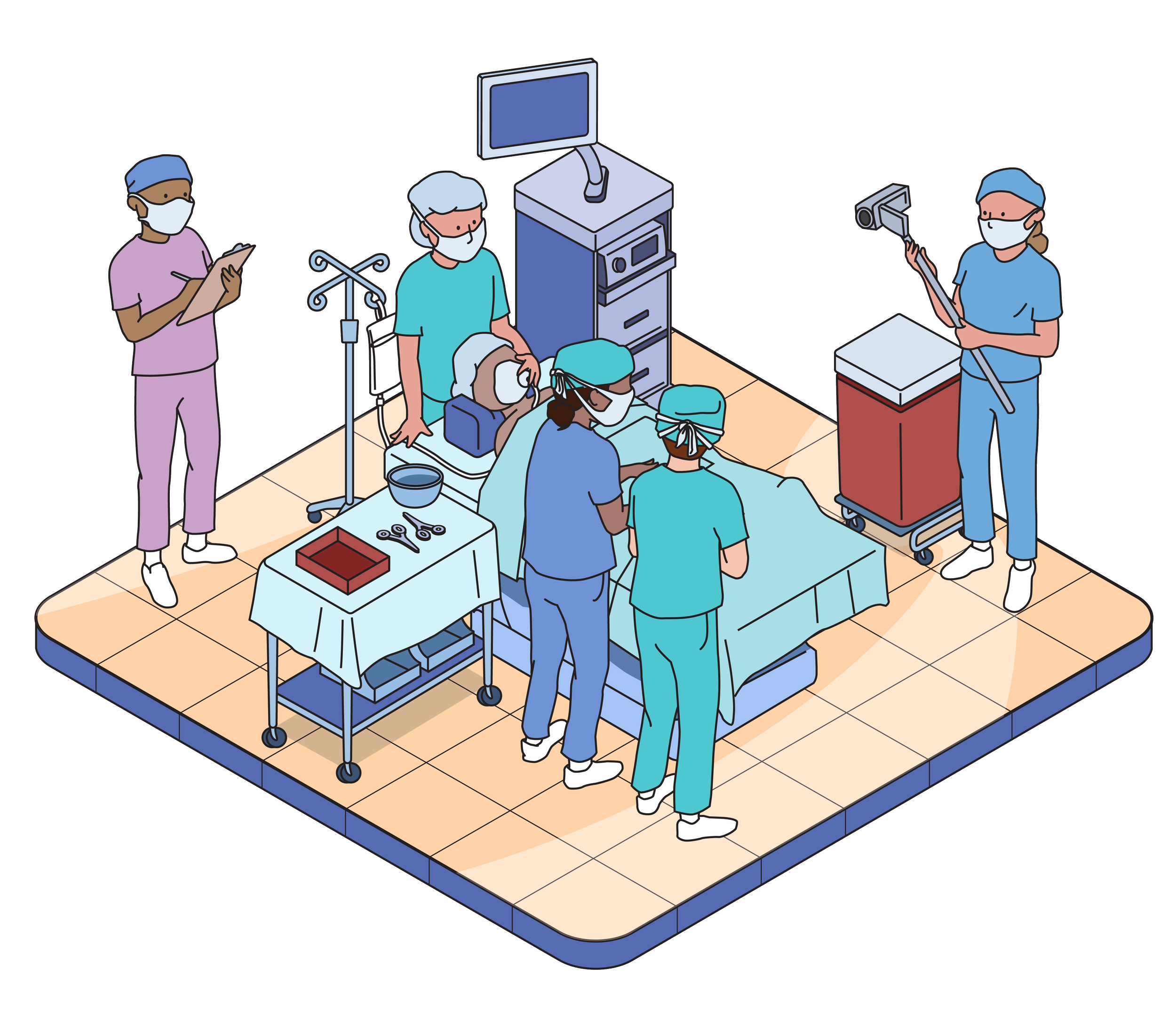
USABILITY TESTING
Usability testing evaluates the usability of products and instructional materials. We do this by observing users simulate use of the product, and will never ask anyone to actually take or inject medication. This testing is not a clinical trial.
EXAMPLES
Formative Studies
Summative/HF Validation Studies
Comparative Use Studies
Recruiting
Moderation
USE-RELATED RISK ANALYSIS
An accurate assessment of use-related risk builds the foundation of every regulatory submission. It is important that risk documentation is built on healthcare industry experience and product knowledge to ensure risks are correctly mitigated and the safety of users is assured. We can help with that!
EXAMPLES
Use-Related Risk Analysis (URRA)
Perception, Cognition, Action (PCA) Analysis
Regulatory Support
Our Labs
We have six state-of-the-art usability labs in Philadelphia and Chicago equipped to replicate any simulated use environment.
Additionally, we have partnerships worldwide to accommodate you or your user populations, wherever they reside.
6
State-of-the-art
usability labs
Testimonials
Our Team
WE WORK HARD TO MAKE AN IMPACT
Our cross-functional team is driven by their passion for understanding “the human factor.”