510(k) Clearance — Byonyks X-1 APD Cycler: Making Dialysis Safer and More Accessible
by Rania Nasif
We are thrilled to share some exciting news from one of our partners, Byonyks – their latest medical device, the Byonyks X-1 Automated Peritoneal Dialysis (APD) Cycler, has received 510(k) clearance from the FDA! This achievement marks a significant step forward in expanding access to life-saving dialysis treatment, and we are proud to have played a role in this journey.
In this post, we will take you behind the scenes of our collaboration highlighting the human factors support we provided, the challenges we faced, the strategies we used, and the impact of our work in helping bring this dialysis therapy to market.
At Design Science, our goal as human factors (HF) experts is to ensure that medical products are safe, effective, and intuitive for the people who rely on them. We conduct usability studies where we observe actual users simulating the use of new or updated medical products to evaluate the usability of the product interface. This work is important because it helps ensure that medical products are safe and easy to use for the people who need them most—patients, caregivers, and healthcare professionals.
Figure 1: Why Usability Testing Matters
The Byonkys X-1 APD Cycler: Designed for Home, Built for Impact
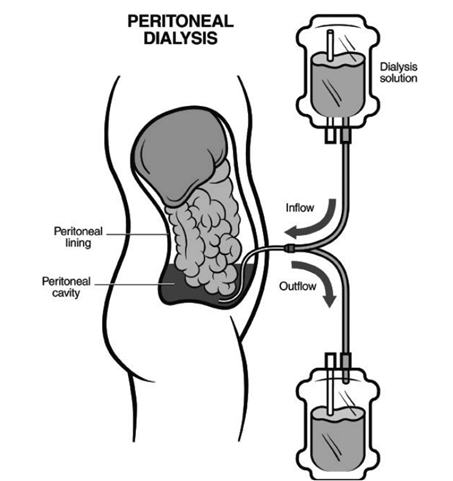
The Byonyks X-1 APD Cycler is engineered to bring automated dialysis into the home, making treatment more accessible and less disruptive to daily life. It works by delivering a special fluid called “dialysate” into the peritoneal cavity—a space inside the abdomen—and then removes it after a set time. This process helps clear waste from the body, a function that the kidneys can no longer perform on their own.
Ensuring Safety and Usability: Our Role in Byonyks’ FDA Clearance
Before most medical devices can be approved for commercial use in the United States, they must undergo a critical step: a human factors validation study. This study is designed to determine if real users can operate the product safely and effectively, as intended.
To navigate this process, Byonyks partnered with Design Science to provide expert HF support. Our team led the planning, design, and execution of their HF validation studies and supported the full submission package to the FDA, helping ensure the product met the necessary usability and safety requirements.
“Special thanks to you and the entire DS team for being there when we needed you badly! Human factors was a big deal! Your flexibility on everything is well appreciated”
Figure 2: Peritoneal Cavity Diagram [3]
Strategy: Flexibility and Global Collaboration
Bringing a medical device to market is a complex endeavor. As an extension of the Byonyks team, Design Science brought fresh thinking to HF strategy and maintained communication flowing smoothly across global time zones.
Flexibility was key. Adapting to evolving regulatory requirements, our team tackled complex challenges in real time. Through FDA discussions, we were able to take a progressive approach to the user groups who participated in the study. Design Science was able to partner with Byonyks to successfully create a rationale for combining patients and caregivers into one user group. Another one of the highlights of this collaboration was our ability to recreate our simulated lab environment right at Byonyks’ facility. While we typically conduct studies in our own state-of-the-art usability labs, designed to simulate both home and clinical settings, this project called for something different. And we delivered, showcasing not just our technical expertise, but also our creativity and commitment to meeting clients where they are.
Design Science is proud to stand by our partners and the work we do, which included supporting Byonyks with their conversations with the FDA, leading to a positive interaction. This global collaboration exemplified how teamwork, innovation, and resilience can successfully drive a complex regulatory process to completion.
Following the multi-year collaboration between Design Science and Byonyks, the FDA issued 510(k) clearance of the Byonyks X-1 APD Cycler in May 2025.
At Design Science, we are proud to collaborate with companies of all sizes to create safer, more effective healthcare experiences. If your team is working on the next breakthrough in medical technology, we would love to collaborate and help make a meaningful difference in patients’ lives. Usability starts here.
Works Cited
[1] Byonyks – A Medical Device Company. (2024). Byonyks.com. https://byonyks.com/
[2] Bassuner J, Kowalczyk B, Abdel-Aal AK. Why Peritoneal Dialysis is Underutilized in the United States: A Review of Inequities. Semin Intervent Radiol. 2022 Feb 18;39(1):47-50. doi: 10.1055/s-0041-1741080. PMID: 35210732; PMCID: PMC8856784.
[3] PERITONEAL DIALYSIS What You Need to Know. (n.d.). https://www.kidney.org/sites/default/files/11-50-0215_peritonealdialysis.pdf
Share this entry